About Us
Syner-G BioPharma Group is a trusted development partner applying innovative and scientifically driven approaches to drug development. The entire Syner-G organization is built around the premise of guiding small molecule, biologics, and cell and gene therapy innovators through drug development, from pre-approval through post-approval, via the application of our integrated services including CMC support, medical writing, regulatory strategy and regulatory operations. Our industry leading solution, CMC 360™, is a fully integrated suite of CMC solutions that encompasses pharmaceutical development, regulatory services, quality/cGxP compliance, and IT systems.
Since its founding in 2007, Syner-G has enabled clients in their quest to bring life-saving and life-enhancing products to patients. Today, the company has grown to more than 200 employees globally, been recognized as one of the “Top 10 Drug Development and Consulting Services Companies” by Pharma Tech Outlook, and served as an integral part of more than 500 various types of successful regulatory filings. Our ongoing expansion across the globe allows us to provide consistent high-quality services in all geographies, further differentiating the company from typical consulting firms.
History
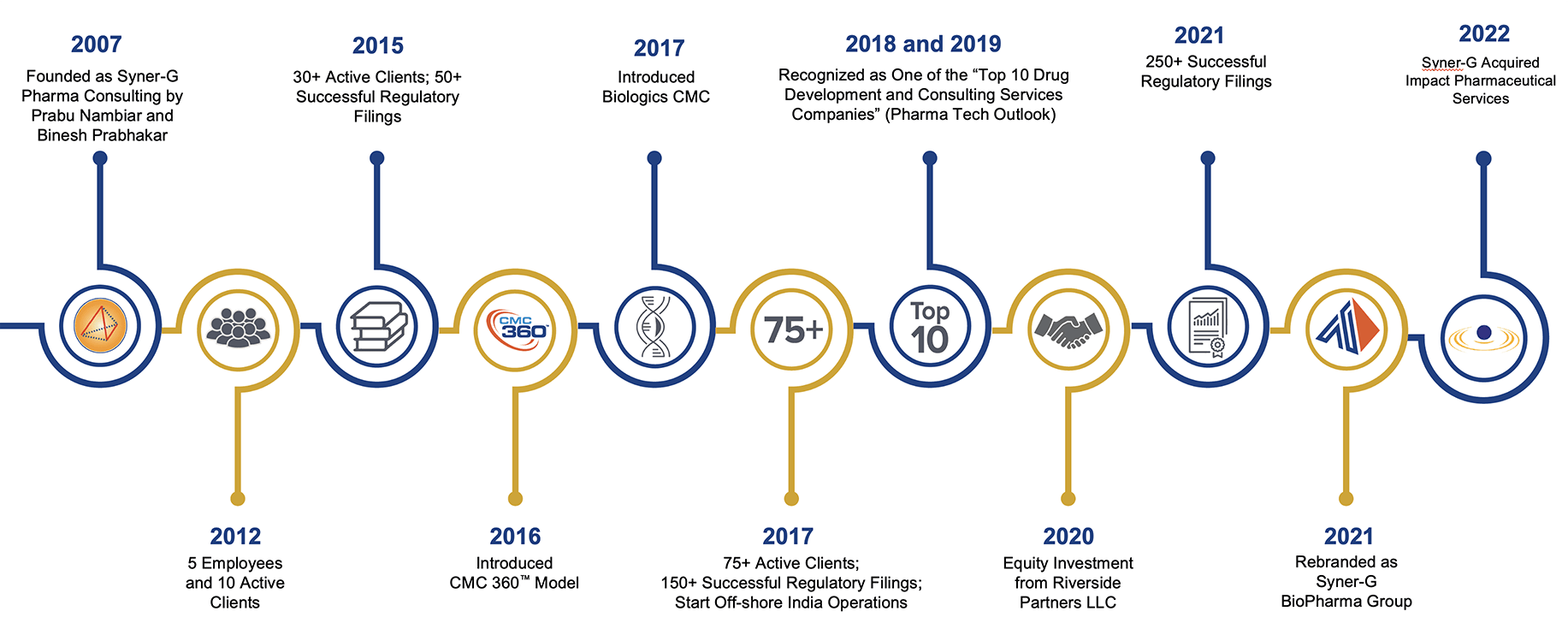
In 2022, Syner-G acquired Impact Pharmaceutical Services. Based in Research Triangle Park, NC, Impact supports the outsourced medical writing, regulatory strategy, and regulatory publishing and submission needs of biotech and pharma companies from pre-IND through post-marketing of drugs and biologics. The integration of Impact added new capabilities to Syner-G, notably in the areas of medical writing, overall drug development and regulatory strategy, and regulatory publishing and submission services. Collectively, the new, larger organization is better suited to support customers in their quest to develop lifesaving and breakthrough therapeutics.
Syner-G BioPharma Group has its headquarters in the greater Boston area, along with offices in Research Triangle Park and India. Reach out to us to start the conversation.
Headquarters
100 Pennsylvania Avenue, Suite 310
Framingham, MA 01701
Contact Us
508.460.9700
info@synergbiopharma.com
